Tissue processing is a sequential procedure vital for preparing biological samples for microscopic study. This article introduces the fundamental steps involved in this process. With a particular focus, we will then explore the critical importance of fixation, the initial step that lays the foundation for successful tissue preservation and accurate histological interpretation.
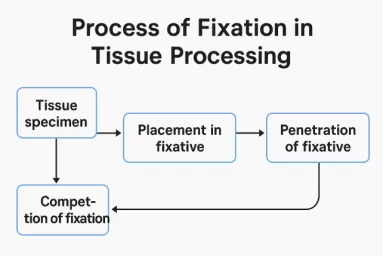
Tissue processing is a crucial step in histology that prepares tissue specimens for microscopic examination by converting fresh tissue into a form that can be easily sectioned and stained. The goal is to preserve the tissue’s structure and cellular detail, making it suitable for accurate diagnosis and research. There are several several key steps in the whole process. Each step in tissue processing is vital to preserving the sample’s microscopic detail and ensuring accurate pathological evaluation.
Fixation
This is the first and most important step where tissues are preserved to prevent decay and maintain structural integrity. Common fixatives like formalin stop enzymatic activity and bacterial growth, helping to stabilize the tissue for further processing.
Dehydration
Water is removed from the tissue using increasing concentrations of alcohol. Since embedding media like paraffin are not water-soluble, complete dehydration is necessary to prepare the tissue for the next steps without distortion.
Clearing
Alcohol in the tissue is replaced with a clearing agent, usually xylene or a xylene substitute, which is compatible with both alcohol and paraffin. Clearing makes the tissue translucent and prepares it for infiltration.
Infiltration
The tissue is saturated with molten paraffin wax. This process gives the tissue enough firmness to be sliced into ultra-thin sections for microscopic analysis without losing its internal structure.
Embedding (Post-Processing Step)
After processing, tissues are embedded in paraffin blocks. This step aligns and orients the tissue correctly before it is sectioned on a microtome and mounted onto slides.
Fixation is the first step and plays a vital role in preserving the tissue’s structure and preventing degradation. It involves the use of chemical agents—commonly formalin (formaldehyde)—to halt all biochemical reactions and stabilize cell and tissue structures. Why Fixation Is So Important?
Prevents Autolysis and Putrefaction
Cells contain enzymes that can break down tissues (autolysis) shortly after death. If not fixed quickly, these enzymes cause structural loss. Fixation halts these processes. It also prevents bacterial growth (putrefaction), which further protects tissue integrity.
Preserves Morphology
Proper fixation maintains the normal appearance and relationship of cells and tissues. It ensures that diagnostic features like nuclei, cytoplasm, and extracellular matrix remain clearly visible under the microscope.
Enables Reagent Penetration
Well-fixed tissue allows reagents (like alcohol and paraffin) to penetrate effectively during later stages. Poorly fixed tissues may have blocked diffusion channels, leading to incomplete dehydration, clearing, and infiltration.
Supports High-Quality Staining
Many histological stains, such as Hematoxylin and Eosin (H&E), rely on good fixation to produce clear, crisp, and interpretable staining. Improper fixation can result in weak, uneven, or false staining outcomes.
Irreversible Impact
Unlike later steps (e.g., dehydration or clearing), errors in fixation cannot be corrected. Once a tissue is under-fixed or over-fixed, its structure may be permanently altered or destroyed, affecting the entire diagnosis process.
Fixation is the foundational step in tissue processing, serving to preserve tissue specimens in as close to a life-like state as possible. Once a tissue is removed from the body, it begins to degrade rapidly due to enzymatic activity (autolysis) and bacterial invasion (putrefaction). To prevent this, the tissue must be quickly placed in a fixative solution—commonly 10% neutral buffered formalin. This chemical stabilizes proteins and cellular structures, effectively “locking” the tissue in its current state to prevent decay and maintain morphology for microscopic evaluation.
Before fixation, the tissue is typically trimmed into smaller, thin sections—usually not thicker than 3–5 millimeters. This ensures the fixative can penetrate the entire sample evenly and efficiently. The volume of fixative used is also important; it should be at least 10 to 20 times the volume of the tissue. Once immersed, the specimen remains in the fixative for a specified period—often between 6 and 24 hours—depending on the tissue type, size, and desired level of preservation. Under-fixation may leave tissue vulnerable to breakdown, while over-fixation can cause hardening or masking of cellular details.
After fixation, the tissue may be rinsed to remove excess fixative and prepare it for subsequent processing steps such as dehydration, clearing, and paraffin infiltration. Proper fixation not only preserves structural detail but also significantly affects the quality of staining and ease of sectioning. Because all downstream processes depend on well-fixed tissue, fixation is considered the most critical step in tissue preparation for histological analysis.